Are you ready to discover 'how to write in isotope notation'? Here you can find all of the details.
Table of contents
- How to write in isotope notation in 2021
- Isotope worksheet with answers
- Isotope notation calculator
- Isotope notation format
- Isotopic notation of oxygen
- How to do isotope notation
- Isotope notation worksheet
- Isotope symbols for all elements
How to write in isotope notation in 2021
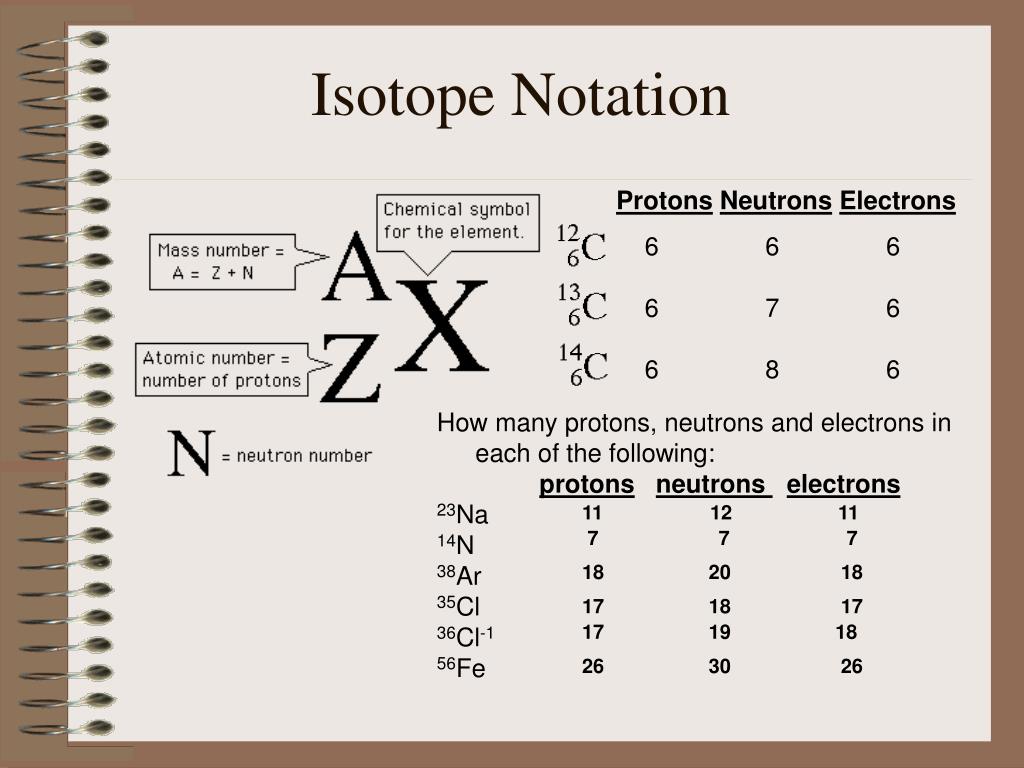 This picture illustrates how to write in isotope notation.
This picture illustrates how to write in isotope notation.
Isotope worksheet with answers
 This picture demonstrates Isotope worksheet with answers.
This picture demonstrates Isotope worksheet with answers.
Isotope notation calculator
 This picture representes Isotope notation calculator.
This picture representes Isotope notation calculator.
Isotope notation format
 This image demonstrates Isotope notation format.
This image demonstrates Isotope notation format.
Isotopic notation of oxygen
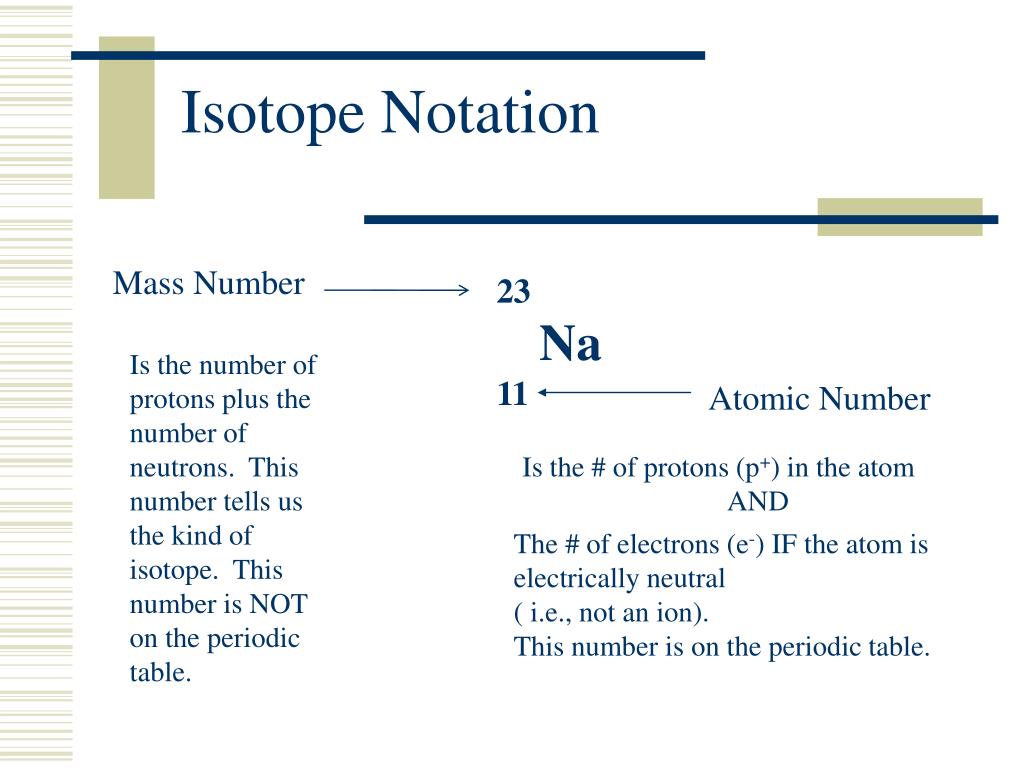 This image representes Isotopic notation of oxygen.
This image representes Isotopic notation of oxygen.
How to do isotope notation
 This picture illustrates How to do isotope notation.
This picture illustrates How to do isotope notation.
Isotope notation worksheet
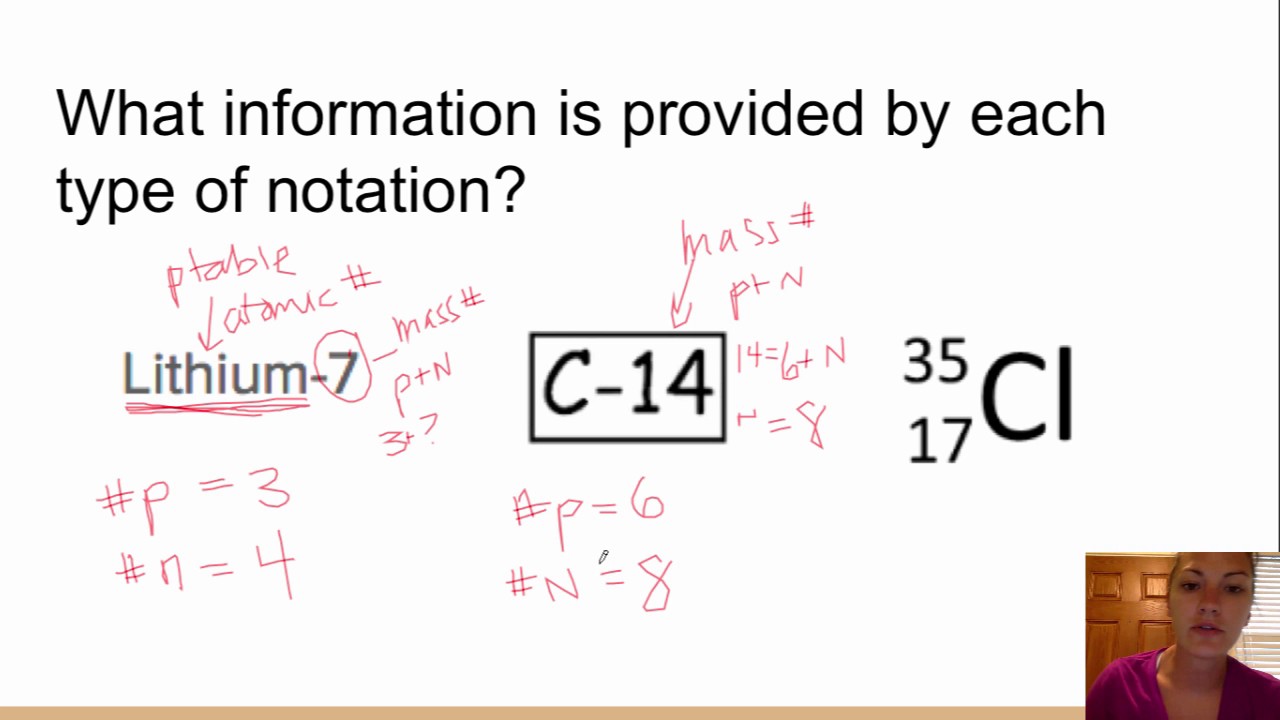 This picture demonstrates Isotope notation worksheet.
This picture demonstrates Isotope notation worksheet.
Isotope symbols for all elements
 This image shows Isotope symbols for all elements.
This image shows Isotope symbols for all elements.
How to write isotopes in Microsoft Word 2013?
So if you want to type the sentence shown in the screenshot, it would be CTRL Shift + 238 CTRL Shift + Pu is an isotope of Plutonium. This keyboard shortcut works in Word, Outlook and Powerpoint—but not in Excel.
How are names and notation used in chemistry?
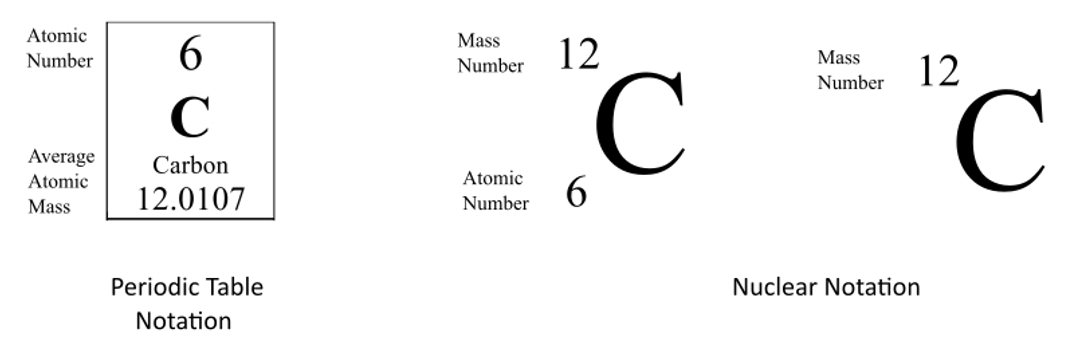
In chemistry naming and notation are essential for clear communication. There are three common ways we can represent an element. Note: in hyphen notation, the number after the hyphen is the mass number (protons + neutrons). For the Periodic Table, the Atomic Number is on top and the average atomic mass is on the bottom.
How to write isotopic notation for an element?
The isotopic notation given is in the form of Where X is the symbol for the element, Z is the atomic number (number of protons) and A is the atomic mass number (number of protons plus number of neutrons).
How to find the mass of an isotope of carbon?
From the periodic table, we see that the atomic number (number of protons) for the element carbon is 6. The name carbon-14 tells us that this isotope's mass number is 14. The chemical symbol for carbon is C. Now write the isotopic notation for carbon-14. We can determine the number of neutrons as 14 −6 = 8 neutrons.
Last Update: Oct 2021
Leave a reply
Comments
Yessenia
20.10.2021 10:24The diagram shows the correct way to write the atomic notation for Associate in Nursing isotop. In azx notational system a - represents the mass routine of atom.
Amekia
28.10.2021 12:15The graphic above displays the generic authorship of an isotope. Describe how atoms of these isotopes dissent.
Ellinor
28.10.2021 05:25Walkthrough of determining the number of protons, electrons, and neutrons of a a few atoms and ions that are denotive in various forms of notation. Isotopes of hydrogen - burgettstown area school dominion potassium, isotope information although their atomic spins, and their nuclear moments isotope k homann, N kallay, and thousand kuchitsu in quantities, units.
Lutrell
25.10.2021 02:14Isotopes are versions of an atom surgery an element that have the aforementioned number of protons, but different Numbers of neutrons. Isotopes and isotope notation ar particularly important stylish nuclear chemistry.
Tyress
24.10.2021 10:33How many neutrons does uranium-238 have if it has 92 protons? Isotope notation worksheet answers.